Novo Nordisk Drops $11 Billion to Expand Weight-Loss Drug Production
The company, now one of Europe’s biggest, is trying to keep supply up with still-astronomical demand.
Sign up for smart news, insights, and analysis on the biggest financial stories of the day.
It’s bulking season at Novo Nordisk.
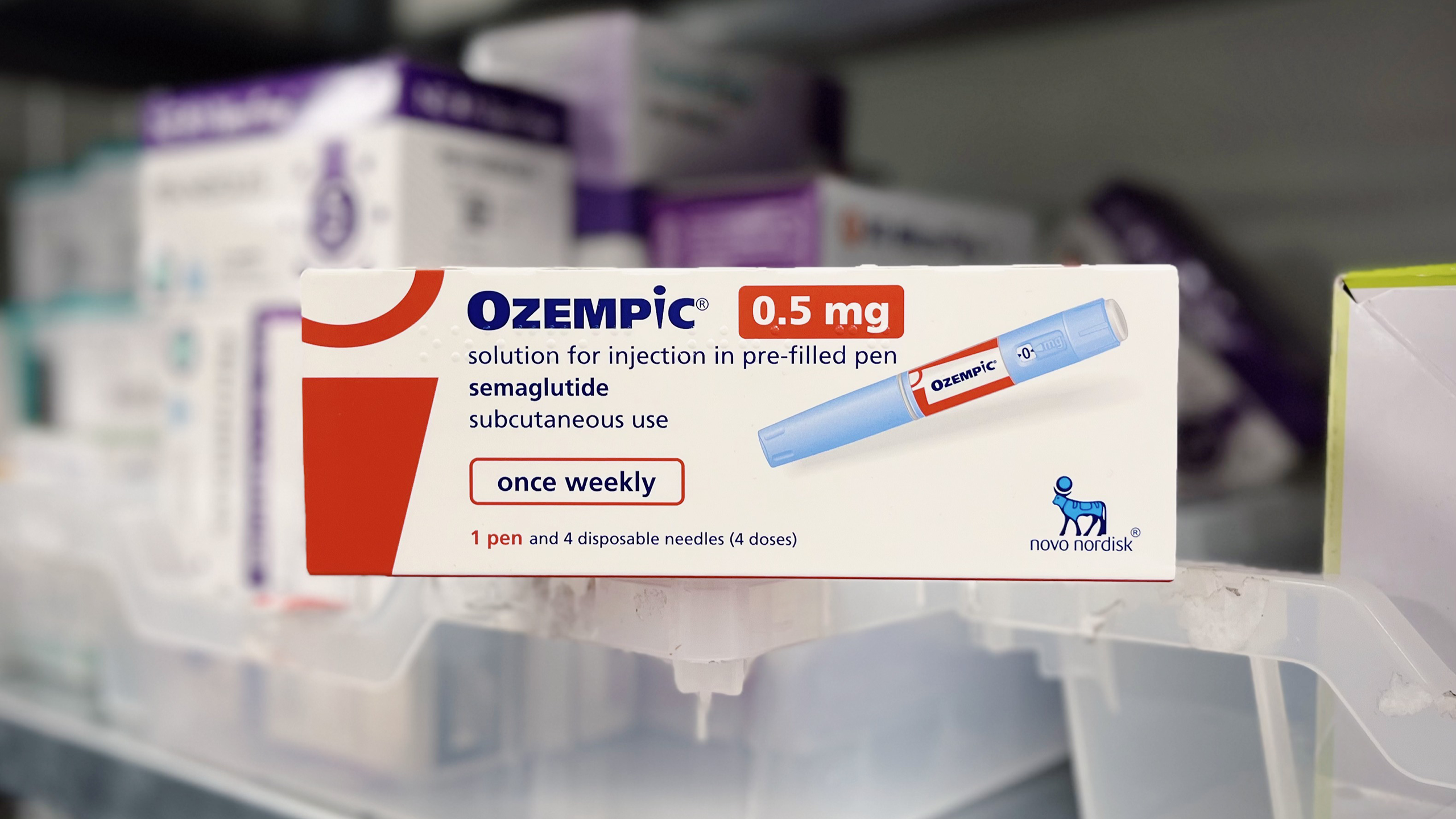
On Monday, the Danish pharma firm behind Wegovy and Ozempic announced an $11 billion deal to significantly expand production capacity for its blockbuster weight-loss drugs.
The Weighting Game
The massive success of both drugs almost instantly made Novo Nordisk the biggest company in Europe. Last week, it announced better-than-expected earnings for 2023, with operating profit up 37% in Danish kroner and its sales projections for 2024 showing another revenue increase of up to 24%. It was enough to send its market cap over $500 billion.
But Novo Nordisk also warned of supply chain constraints strangling the company’s ability to keep up with soaring demand. On Monday, Novo showed it’s ready to throw its prodigious new weight around:
- In a semi-complex three-way transaction, Novo Holdings — Novo Nordisk’s controlling shareholder — is purchasing US drugmaker Catalent for $16.5 billion. Novo Holdings is then selling three of Catalent’s manufacturing sites, in Italy, Belgium, and Indiana, to Novo Nordisk for $11 billion.
- Novo Nordisk had already contracted part of Wegovy’s production process to Catalent, which is known for its role in “fill-finish” operations in the final stages of manufacturing for injectables such as Wegovy; the company had previously contributed to covid vaccine manufacturing for Moderna, AstraZeneca, and Johnson & Johnson.
Novo Nordisk said it expects the deal to be completed by the end of the year, and the additional manufacturing prowess to help expand its production capabilities by 2026.
Rat Race: Keeping up with demand isn’t solely a Novo Nordisk problem. Also on Monday, Mounjaro, Eli Lilly’s blockbuster diabetes drug which has doubled as an off-label weight loss drug, was added to the FDA’s drug shortage list due to surging demand surpassing the company’s supply. We’ll chalk it up to New Year’s weight loss resolutions.